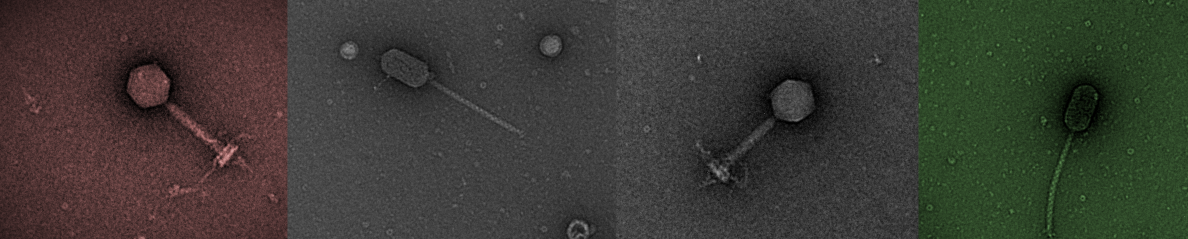
As part of an undergraduate teaching project, we have recently sequenced a number of bacteriophage genomes. At the end of the sequencing analysis is a submission to a database prior to publication. There are several databases to which newly sequenced genomes can be submitted: EBI, NCBI and DDJB . My preferred database is EBI, frankly because the submission process is less painful (relatively) than using NCBI and receiving an accession number of the submitted genome takes less time. Having gone through it several times recently, I thought it would be a good idea to outline the steps required to complete the submission in the least painful and time-consuming way.
In order to complete a submission process, you need several things:
- Webin account
- A complete annotated genome (gzipped),
- Raw Fastq reads (gzipped),
- TaxonID code
- Phage name
- Locus tag
- Library insert size
- Covearge of the genome
- Description of the sequencing technology and the machine used . eg NexteraXT with a MiSeq
- The relevant metadata for the phage , eg data of isolation, source of isolate etc
If you haven’t re-sequenced a previously sequenced bacteriophage, then a new Taxon ID code is required for your newly sequenced phage. This code has to be requested and needs to be done in advance of submission (you can start the submission, but will not be able to get very far without the Taxon ID). For a new Taxon ID a PROJECT ID is needed. So the first step in submitting a genome is to create a new PROJECT under ENA Webin submission (If you dont have a Webin account you will have to create one first). During this process, you can also reserve or create a unique locustag for the genome.The process of requesting a new Taxon ID only takes few day and needs to be done in advance of submission, as you won’t get far in the submission process without it. Here is a brief list of requirements for the Taxon ID request::
Full details of how to get a new Taxon ID can be found here
Once these have been created then the genome can be annotated and formatted correctly for submission. A range of programs can be used for genome annotation, a good option for the first pass is PROKKA . Using the “–locustag XXXX” option with PROKKA will automatically create the locus tags with correct locus identifier if XXXX is replaced by the locus tag created/reserved when the project was created. eg
prokka1.11 —locustag XXXX —addgenes contigs.fasta –prefix phage1 —outdir phage1
Running this command, will create a file called phage1.gbk that we can use for the basis of submission. The next step is to change the format of this file to EMBL format. For this, I use a script called genbank_2_embl.pl to create a file called phage1.embl.This conversion using BioPerl creates a file with the following few header lines:
ID unknown; SV 1; linear; unassigned DNA; STD; UNC; 348043 BP.
XX
AC unknown;
XX
XX
XX
OS Genus species
OC Unclassified.
XX
CC Annotated using prokka 1.11 from http://www.vicbioinformatics.com.
XX
Unfortunately, these few lines are not correct for submission in the current form and need to be changed to meet current flat file standard. The above lines outlined in red need to be changed to meet the current requirements. Therefore those lines need to be changed to look like this. The ID line should only include linear or circular , depending on the type of phage you have. PR, contains the project ID that is obtained when the study is registered. Full details of what is needed can be found here
ID XXX; XXX; linear; XXX; XXX; XXX; XXX.
XX
AC XXX;
AC * _{chr1}
PR Project:PRJEB1234;
XX
RL Submitted (17-JUL-2016) to the INSDC.
XX
XX
XX
XX
CC Annotated using prokka 1.11 from http://www.vicbioinformatics.com .
I did this using a script called emb_reformat.pl .The line PR should contain the PROJECT ID that was generated earlier. This file can now be checked with the validator program for flatfiles from EBI that can be obtained here.
This will identify any issues with the files prior to submission that will need to be fixed. The validator program output file VAL_ERROR.txt contains the details of any errors and give clues to how they may be fixed. Once these reports have no errors the final EMBL file can be gzipped ready for submission. This is easily done with the command gzip.
One of the additional requirements for submission is a md5sum value. More information on what MD5SUM values are and why they are used can be found here. MD5SUM value of the gzipped filw embl1.gz file can be produced using the following command line:
md5sum embl1.gz
MD5SUM values will also be needed for the gzipped fastq files. These values can be obtained in a similar manner to the above example for the embl file.
Submission also requires the insert size of the library and the coverage of the genome. There is a number of ways these parameters can be calculated. It is highly likely that in the process of genome assembly, a BAM file has been generated with reads being mapped back to the assembled phage genome. We can use a program called Qualimap to analyse the produced BAM files and get the insert size and the coverage. In order to make this process easier for multiple genomes, I use the following perl scripts calc_bam_coverage.pl and calc_bam_insert.pl
The final checklist of things that are needed are
-
Annotated phage genome gzipped and md5sum value
-
Raw fastq files gzipped and md5sum values
-
TaxaID value
-
Insert size of library
-
Coverage of genome
-
Assembly algorithm used
- Sequencing platform used
Once you have this information it is relatively painless (note relatively) to submit a single genome to EBI. As you have created a PROJECT ID already to get a locus tag and Taxon ID, there is no need to create another project. I normally submit reads first, then my assembly and in the process they are all associated with the same PROJECT ID.
Upon submission of the assembly files, there is a bewildering number of combinations of choices of scaffolds/contigs/chromosomes and annotated or not. Most bacteriophages will assemble into a complete genome without gaps or NNNs. There the choice would be
Does the assembly contain contigs? NO
Does the assembly contain scaffolds? NO
Does the assembly contain chromosomes? YES
Are the chromosomes functionally annotated? YES
These choices are what I have been advised to use by a member of the ENA Team.
This therefore requires a Chromosome list file ( and the MD5SUM of it ). This format of this file is very simple and the detaisl of how it can be produced arehere
In its simplest form, it is a one line tab delimited file . eg
chr01 I Chromosome